Our Core Businesses
We focus on innovative product R&D and structuring of product development pipelines in four business areas:
biologics, small molecules, synthetic biologics, and traditional Chinese medicines (TCMs).
R&D Centers
We have set up five product R&D centers in China and the U.S., preliminarily built a qualified team and an international R&D system meeting practical requirements,
and established a perfect supporting team responsible for clinical trial and registration applications.
The supporting team can undertake the whole process of new drug research from project evaluation and initiation, new drug screening, API and preparation technology research
to quality standard formulation, clinical research, registration and submission for approval, so as to promote early marketing of the products under research.
-
800+
R&D people
-
~200
MS and PhD holders
-
~300
International/domestic patents
-
~20
PCT patents
R&D Platforms
-
-
-
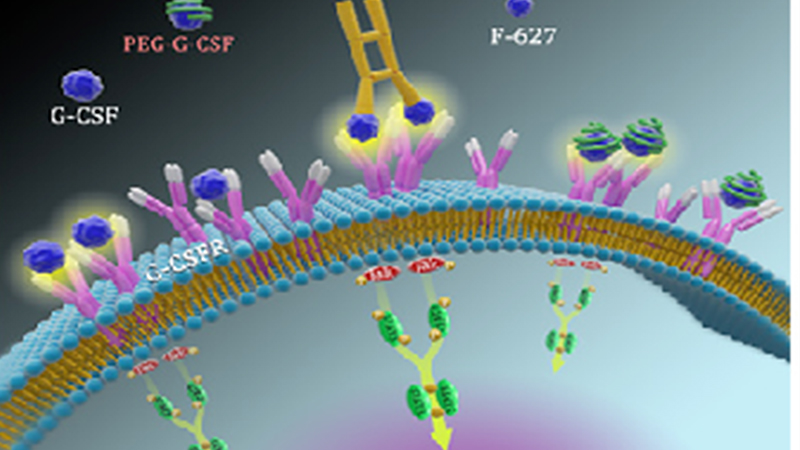
Macromolecular biologics innovation platform
The Macromolecules Division possesses an innovative drug development platform based on both eukaryotic expression and prokaryotic expression systems. Di-Kine bimolecular platform and LaMbs long-acting double-antibody platform are two eukaryotic expression platforms owned by the Macromolecules Division. In addition, the Division also possesses multiple biologics R&D platforms based on prokaryotic expression systems, including oral polypeptide platform, long-acting polypeptide platform and nano antibody technology platform.
The Di-Kine platform uses the unique Fc fusion protein dimer technology to significantly extend the circulating half-life of drugs in the body. This platform is especially suitable for the development of long-acting products of endogenous cytokines, hormones, etc. The products developed are closer to the folding and modification of its endogenous counterpart, and have better efficacy and safety. Phase III clinical studies for the innovative drug product F-627 developed based on this platform has been completed in China, Europe and the U.S., and marketing applications have been filed in the three places.
On the basis of the unique double-antibody design technology, the LaMbs long-acting double-antibody platform has great development flexibility and is suitable for the development of multi-target double-antibody products. This platform will be mainly used in product development for non-tumor indications. Products developed based on this platform are currently in the preclinical stage.
By locating a reasonable delivery site and designing a drug release system, the oral polypeptide platform can reduce the degradation by gastrointestinal enzymes and promote drug absorption, thereby making oral administration of such drugs possible, avoiding discomfort of patients caused by injection administration, and increasing compliance with drug administration.
On the basis of fatty chain modification and fusion protein technology, the long-acting polypeptide platform can enhance the combination with human serum albumin, and prolong the half-life of polypeptide drugs, thereby realizing long interval administration of polypeptide drugs.
Taking advantage of small molecular weight, good tissue permeability and flexible molecular assembly design of nano antibodies, the nano antibody platform is used for the development of antibody preparations for respiratory system and external use, as well as bi-specific and multi-specific antibody products.
At present, the Macromolecules Division has filed more than ten PCT applications related to new products on the basis of the innovative drug development platform.
-
-
-
-
Synthetic biologics platform
The platform has focused on the field of biological fermentation and enzyme catalysis for more than 20 years, and has laid a solid foundation for the enzymatic-chemical combination process and industrial scale-up technology. To efficiently promote the projects under research, the platform has made full use of the scientific research advantages of other scientific research institutions, and has been in partnership with Jiangnan University and Zhejiang University of Technology for many years. In addition, the platform has maintained long-term close cooperation with Amryis,and other top synthetic biologics companies both at home and abroad.
The platform takes yeast, Escherichia Coli, etc. as chassis microorganisms, and sugar as the main raw material to synthesize various vitamins, intermediates and pharmaceutical and nutritional products. The whole process integrates construction of genetic engineering bacteria, large-scale high-density fermentation, metabolic regulation, directed evolution of enzymes, construction and enhancement of high substrate concentration catalytic system, separation and refinement of products. The platform has possessed a complete and efficient industrial scale-up technology system of synthetic biologics.
-
-
-

-
Chemical synthesis platform
Focusing on the treatment of blood tumor, first aid, children and rare diseases, the chemical synthesis platform has built raw material technology platforms such as innovative drugs and intermediates, nucleoside drug synthesis, solid phase peptide synthesis, and special excipients, as well as preparation technology platforms integrating R&D and production, such as oral slow controlled release preparations, micron suspension, micelles for injection, solubilization of insoluble drug, children's appropriate preparations, and high-end complex liquid preparations.
The platform has world-class process equipment and testing equipment, such as microchannel reactor, preparative chromatography, multifunctional fluidized bed, dry granulator, airflow grinder, flow-through-cell dissolution tester, temperature-controlled low-viscosity viscometer, micrometer and nanometer laser particle sizer, vacuum attenuation tester, LC-MS analyzer and thermal analyzer, which meet the research needs of innovative and generic raw materials and preparations. In addition, the platform has established close cooperation with scientific research institutes such as the University of Science and Technology of China, the China Pharmaceutical University, and East China University of Science and Technology. The platform is committed to carrying out innovation, improvement, generic drug R&D and technology industrialization for domestic and international markets.
Ten new products of the platform have been approved, more than 100 projects are under research, and 10~20 new drugs are developed and marketed each year.
-
-
-
-
TCM R&D platform
Focusing on the traditional advantage area of Chinese patent medicines, especially the fields of gynecology, pediatrics, orthopedics, dermatology, etc., the TCM R&D platform has been committed to the development of special TCMs, has launched a series of multi-faceted product lines having special clinical value, and established a relatively complete TCM R&D system. By in-depth innovation of new TCMs, quality improvement of TCMs, R&D of age-old classical prescriptions, secondary development of large varieties, and technological innovation in internationalization of TCMs and other aspects, the TCM R&D platform has gradually grown into a technical platform for R&D of new TCMs, and basic and applied research of TCM, such as key technologies of TCM industry and new TCMs.
The platform possesses first-class TCM preparations and testing equipment, such as wet granulator, multi-function fluidized bed, dry granulator, freeze dryer, airflow grinder, HPLC and other modern large-scale instruments and equipment, which can fully meet the research needs of innovative TCMs and cultivation of large varieties. The platform has established close cooperation with Beijing University of Chinese Medicine, China Pharmaceutical University, and Tianjin Institute of Pharmaceutical Research, among other scientific research institutes, with a view to carrying out innovation, R&D of large varieties and health care products and technology industrialization for both domestic and international markets. At present, more than 40 projects of the platform are under research, including more than 10 new drug R&D projects, and more than 30 projects for technology improvement and secondary development. For the moment, the platform has obtained 2 approvals for clinical study of new drugs (both under clinical trial research), 1 approval for protected variety of TCM, and 1 overseas listed TCM product. In addition, the platform has applied for more than 30 national patents, including more than 10 authorized patents.
-